{{ item.AUTOREN_ZITAT }}:
{{ item.TITEL }}{{ zitatInLang(item) }}

PhD Students
Master Students
Exchange Students

Former Group Members

Twitter@LabWaser, opens an external URL in a new window
April 1st, 2024: Paul Joël Henry (Rouen, France) oins our group as a PhD student.
March. 4th, 2024: Our paper reporting the ITU-catalyzed asymmetric (4+2)-cycloaddtions of allenoates with different Michael acceptors has been accepted for publication in Adv. Synth. Catal.
Feb. 1st, 2024: Gabriel Burel (Rouen, France) joins our group as an exchange Master student.
Jan. 7th, 2024: William Nzegge joins our group as a PhD student.
Dec. 28th, 2023: Our paper reporting asymmetric ammonium salt-catalyzed Michael addition reactions of hydantoins has been accepted for publication in a special issue dedicated to Prof. Fürstner in Helv. Chim. Acta.
Nov. 27th, 2023: Our paper reporting the ITU-catalyzed activation of allenoates for asymmetric (4+2)-cycloaddtions has been accepted for publication in Angew. Chem..
Oct. 19th, 2023: Two papers, one reporting a synergistic ITU/Pd-catalysis protocol to access d-lactams and the other one reporting oxidative a-thiocyanations and a-selenocyanations of different pronucleophiles have been accepted for publication in the European Journal of Organic Chemistry each.
Oct. 1st, 2023: Behzad Nasiri and Ghaffar Pasdar join our group for a 6 months research stay.
Aug. 25th, 2023: Our paper reporting the ITU-catalyzed synthesis of e-lactones using novel p-quinone methide building blocks has been accepted for publication in the European Journal of Organic Chemistry.
Aug. 11th, 2023: David Naderer successfully defends his Master thesis. He will stay in our group to carry out future PhD studies.
June 12th, 2023: Our paper reporting the counter anion-directed ammonium salt catalyzed oxidative azidation of phenols has been accepted for publication in a special collection on iodine chemistry in Advanced Synthesis & Catalysis.
April 25th, 2023: Our paper reporting the photochemical Wolff rearrangement initiated generation and α-chlorination of ammonium enolates has been accepted for publication in Organic Letters.
April 18th, 2023: Our collaborative paper with Andy Smith's group (St. Andrews) reporting the chiral Lewis base-catalysed activation of glycine Schiff base conjugate additions has been accepted for publication in the newly established journal ChemistryEurope.
March. 27th, 2023: Mario Hofer and David Pollheimer join the group to carry out their master theses.
Feb. 16th, 2023: Sajid Jahangir (Prof. at the Federal Urdu University of Art, Science, and Technology, Karachi, Pakistan) joins us for a 3 months research stay as an OEAD fellow.
Feb. 1st, 2023: Meysam Aryafard (University of South Bohemia) joins the group as an Erasmus exchange PhD student.
Jan. 20th, 2023: Magdalena Piringer successfully defends her Master thesis. She will stay in our group to carry out future PhD studies.
Sept. 29th, 2022: Roman Schütz successfully passes his Diploma examination. All the best for your future, Herr Lehrer!!
Sept. 21st, 2022: Our paper reporting the ammonium salt-catalyzed b-addition of isoxazolidin-5-ones to allenoates has been accepted for publication in a special issue dedicated to Prof. Cristina Nevado in Synthesis.
Sept. 20th, 2022: Our paper discussing the organocatalytic synthesis of masked b-amino acid containing phthalides has been accepted for publication in a special issue dedicated to Prof. Janine Cossy in Helv. Chim. Acta.
Sept. 19th, 2022: A perspective article on asymmetric organocatalysis together with Olga Garcia Mancheño from Münster was accepted for publication in Eur. J. Org. Chem.
Aug. 25th, 2022: Our personal account discussing our 10 year journey in bifunctional ammonium salt catalysis has been accepted for publication in a special issue on asymmetric organocatalysis in Chem. Rec.
Aug. 8th, 2022: The Austrian Science Funds FWF grants a new stand alone project allowing us to focus on new aspects in allenoate chemistry. Generous financal support comes from the matching funds initiative between the FWF and the state department of Upper Austria!
June 13th, 2022: Victoria Haider successfully defends her PhD thesis and will soon start working for Takeda Linz. Was wonderful having you in the group Vicky and all the best for your future.
April. 26th, 2022: Lukas Vogl successfully defends his Master thesis. He will stay in our group to carry out future PhD studies.
March 29th, 2022: Our paper describing the ammonium hypoiodite-catalysed decarboxylative synthesis of dihydrobenzofurans has been accepted for publication in Org. Biomol. Chem.
Feb. 21st, 2022: Lorenzo Serusi from Antonio Massa's group in Salerno, I, joins us for a 6 months research stay in our group.
Jan. 10th, 2022: Daniel Chrenko from Jiri Pospisil's group in Olomouc, CZ, joins us for a 3 months research stay in our group.
Jan. 3rd, 2022: Our full paper describing the asymmetric synthesis of a-selenated masked a- and b-amino acid derivatives has been accepted for publication in Org. Biomol. Chem.
Since 11/2021: Head of the Institute of Organic Chemistry, JKU Linz.
Since 01/2020: Full Professor for Organic Stereochemistry at the Institute of Organic Chemistry, JKU Linz.
11/2014-12/2019: Associate Professor at the Institute of Organic Chemistry, JKU Linz.
06/2014: Habilitation (venia docendi) for organic chemistry - "Design of Tartaric Acid-Derived Chiral Organocatalysts and Development of Stereoselective Reaction Procedures" (Mentor: Prof. Dr. Norbert Müller).
Since 02/2011: Deputy Head of the Institute of Organic Chemistry, JKU Linz.
07/2009-10/2014: Assistant Professor at the Institute of Organic Chemistry, JKU Linz.
04/2007-06/2009: R&D Chemist DSM Fine Chemicals Austria, Linz.
02/2006-04/2007: Postdoc at the Max-Planck Institut für Kohlenforschung (Mülheim/Ruhr-Germany) in the group of Prof. Dr. Alois Fürstner, FWF - Erwin Schrödinger fellowship J2586 - "Totalsynthesis of the Iejimalides".
10/2003-10/2005: PhD thesis at the Institute of Organic Chemistry, JKU Linz in the group of Prof. Dr. Heinz Falk - "9,12-Disubstituted Hypericin Derivatives as Potential Second Generation Hypericin Based Photosensitizers" (FWF-Project P16969).
03/2003-09/2003: Diplomathesis in DSM Fine Chemicals Austria Linz under the supervision of Prof. Dr. Heinz Falk "Transition Metal Catalysed Oxidation of Benzylic Compounds using Ozone".
09/1998-09/2003: Student of WITECH (Technical Chemistry with additional basics in economics) at the Johannes Kepler University Linz.
27/09/1977: Born in Steyr, Austria
03/2024: Outstanding Reviewer for Org. Biomol. Chem. in 2023.
03/2024: Outstanding Reviewer for Org. Chem. Front. in 2023.
04/2023: Outstanding Referee for Angew. Chem. in 2022.
03/2023: Outstanding Reviewer for Org. Biomol. Chem. in 2022.
02/2023-02/2027: Standalone project funded by the Austrian Science Funds FWF „Allen(oat)e transformations catalyzed by isothioureas“ (P36004)
12/2022: Best Presentation Award at the 26th International Electronic Conference on Synthetic Organic Chemistry.
08/2022: EurJOC Lecture at the Blue Danube Symposium on Heterocyclic Chemistry (Bratislava).
03/2022: Ranked 2nd in the 2021/22 AG Linz "Vote Your Prof" student poll for the Faculty of Science and Engineering at the JKU.
03/2021: Outstanding Reviewer for Org. Biomol. Chem. in 2020.
02/2021: Ranked 1st in the 2020/21 AG Linz "Vote Your Prof" student poll for the Faculty of Science and Engineering at the JKU.
09/2020: Ranked 2nd in the 2019/20 AG Linz "Vote Your Prof" student poll for the Faculty of Science and Engineering at the JKU.
02/2019: Outstanding Reviewer for Org. Biomol. Chem. in 2018.
02/2019: Awarded the "Golden Mitch" (ranked 1st) in the 2018/19 AG Linz "Vote Your Prof" student poll for the Faculty of Science and Engineering at the JKU.
04/2019-09/2024: Standalone project funded by the Austrian Science Funds FWF „Asymmetric a-Functionalization of Carboxylic Acids/Esters“ (P31784)
10/2018: Awarded the "Golden Mitch" (ranked 1st) in the 2018 AG Linz "Vote Your Prof" student poll for the Faculty of Science and Engineering at the JKU.
04/2018: Aarded the "Silver Mitch" (ranked 2nd) in the 2017 AG Linz "Vote Your Prof" student poll for the Faculty of Science and Engineering at the JKU.
03/2018: Outstanding Reviewer for Org. Biomol. Chem. in 2017.
Since 01/2018: Standalone project funded by the Austrian Science Funds FWF „Syntheses of Chiral Fluorinated Amino Acids and Peptides“ (P30237)
11/2015: "Kardinal Innitzer Förderungspreis 2015" (Kardinal Innitzer Young Researcher Award 2015)
09/2014-10/2018: Funding of the Austrian Science Funds FWF of our project: "Syntheses of (Chiral) Hetero- and Carbocycles Using Ammonium Enolates" (P26387)
05/2012: JSP fellowship (Junior Scientists Participation Program) for the 47th Bürgenstock Conference (Brunnen, Switzerland).
02/2011: Selected as an Austrian representative for the 3rd EuCheMs Org Div Young Investigator Workshop in Crete
09/2010-09/2014: Standalone project funded by the Austrian Science Funds FWF: "Novel Tartaric Acid Derived Asymmetric Organocatalysts" (P22508)
01/2006: Erwin Schrödinger fellowship auf the Austrian Science Funds FWF for a Postdoc on the "Totalsynthesis of the Iejimalides" (02/2006 - 04/2007)
10/2004: Poster award CHI´s Discovery on Target – Boston
Since 2021: Member of the International Editorial Advisory Board of Tetrahedron Chem
Since 2021: Member of the International Editorial Advisory Board of Asian Journal of Organic Chemistry
Since 2020: Member of the International Editorial Advisory Board of European Journal of Organic Chemistry
Since 2020: Member of the Editorial Board of Molecules
Since 2020: Member of the Editorial Advisory Board of Monatshefte für Chemie
Since 02/2018: Local GÖCH Chair for Upper Austria
09/2017: Chair of the Organizing Board of the “17th Blue Danube Symposium on Heterocyclic Chemistry” held in Linz (around 170 international participants)
Since 2017: Member of the Editorial Board of Chemical Data Collections
2014-2019: Member of the Editorial Board of Monatshefte für Chemie
2013-2017: Member of the Editorial Advisory Board of Current Organocatalysis
Generous financial support by the following agencies, institutions, and companies is gratefully acknowledged:
Austrian Science Funds (FWF), opens an external URL in a new window
Linz Institute of Technology (LIT)
Upper Austrian Government , opens an external URL in a new window
European Union (Marie Skłodowska-Curie action No 690882), opens an external URL in a new window
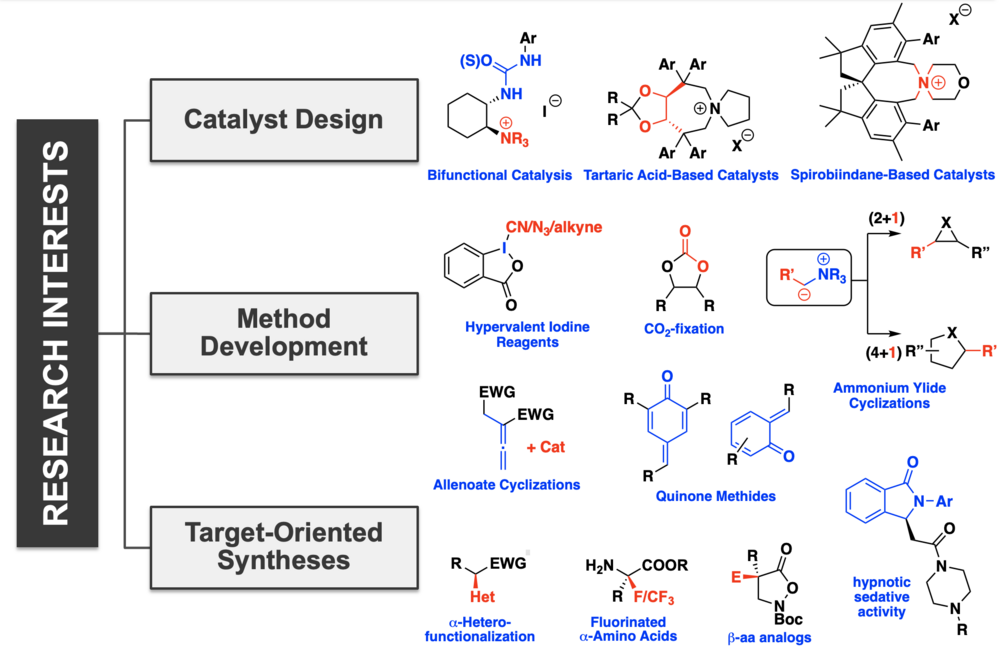
Chiral (Bifunctional) Ammonium Salt Catalysis
Chiral quaternary ammonium salt catalysis is one of the outstanding catalysis principles nowadays. Over the last years our group had a strong focus on the design and development of novel chiral quaternary ammonium salt catalysts based on different chiral backbones as well as catalysts containing an additional catalytically active unit (bifunctional ammonium salts).
New catalysts are investigated and their potential for different applications is thoroughly examined.
Chiral Ammonium Hypoiodite Catalysis
The use of chiral quaternary ammonium iodides under oxidative conditions results in the in situ formation of catalytically active chiral ammonium hypoiodite species. This represents a powerful methodology to merge the potential of asymmetric ammonium salt catalysis and hypervalent iodine chemistry and over the last years some interesting novel approaches utilizing this concept have been reported. Considering our general interest in asymmetric ammonium salt catalysis we are currently very much interested in utilizing these systems under oxidative conditions to develop novel asymmetric target transformations.
LIT Project: „Asymmetric synergistic ammonium (hypo)iodite-imine catalysis“ (LIT-2019-8-SEE-111)
Chiral Lewis Base-Mediated Ammonium Enolate Reactions
In addition to these ion pairing catalysis-based projects we are also interested in exploring the applicability of (a)chiral ammonium enolates for the stereoselective formation of small ring carbo- and heterocycles as well as for asymmetric a-functionalization reactions of simple esters and carboxylic acids. Hereby we focus on chiral auxiliary approaches (i.e. reactions of ammonium ylides) as well as on organocatalytic approaches using chiral nucleophilic catalysis. The hereby accessed structural motifs are highly important as they are often the functional groups of choice in key-intermediates in complex natural product syntheses and therefore we are highly interested in the development of alternative synthesis strategies for such compounds.
FWF Project: „Syntheses of (Chiral) Hetero- and Carbocycles Using Ammonium Enolates“ (P26387)
FWF Project: „Asymmetric a-Functionalization of Carboxylic Acids/Esters“ (P31784)
Chiral Lewis Base-Mediated Allenoate Reactions
Chiral Lewis bases serve well as catalysts for the activation and control of allenoates in cyclization reactions. While such approaches usually rely on phosphines and amines as catalysts, we recently initiated a program focusing on the use of alternative isochalcogenoureas as catalysts for allenoate reactions. Complementary reaction pathways can be accessed giving access to products that are not accessible with existing methods in an asymmetric manner.
FWF Project: „Allen(oat)e transformations catalyzed by isothioureas“ (P36004)
Organocatalytic Methods for the Synthesis of (Fluorinated) Biologically Active Compounds
Our group is also interested in the use of chiral organocatalysts to access compounds or structural motives that are of interest to biological or medicinal chemists, either because of their biological properties themselves, or because of their versatility to serve as key-intermediates in the synthesis of biologically active target molecules. Hereby we have a target-driven approach as well as interest in the development of new methods to access such molecules in a more efficient way. Special focus hereby is on the development of asymmetric fluorination methods towards chiral fluorinated amino acids and peptides.
FWF Project: „Syntheses of Chiral Fluorinated Amino Acids and Peptides“ (P30237)
Marie Skłodowska-Curie action No 690882 "Quatsalts"
Asymmetric a-Functionalization of Carboxylic Acids/Esters, opens an external URL in a new window
(FWF stand alone project P31784, since 04/2019)
The direct a-functionalization of simple acyclic carboxylic acid derivatives is a challenging task and this project aims on the development of new strategies to carry out such reactions in an asymmetric fashion. We will hereby rely on the use of chiral Lewis base catalysts which provide a highly reactive chiral ammonium enolate intermediate upon reaction with simple starting materials. This intermediate will then undergo the targeted asymmetric a-hetero- and carbofunctionalization reactions. The hereby accessed enantioenriched products are of high value for further applications and therefore this project will provide powerful tools to overcome challenging synthesis problem and will give access to a variety of interesting chiral small molecules in a so far unprecedented and highly efficient manner relying on simple starting materials.
Project Publications:
Please see publications 77, 79, 80, 81, 82, 83, 85, 87, 88, 89, 90, 94, 99, 100, 102, 104 and 109 from our general publication overview for further details about the research carried out in the course of this project.
Allen(oat)e transformations catalyzed by isothioureas, opens an external URL in a new window
(FWF stand alone project P36004, since 02/2023)
Allenoates represent a class of easily accessible and highly reactive small compounds that allow for unique reactions in the presence of different catalysts. With respect to the catalyst motives of choice, the field is clearly dominated by (chiral) tertiary phosphines and tertiary amines. Very importantly, the nature of the used catalyst has a pronounced effect on these reactions,which often allows for orthogonal reactivities and connectivities when utilizing different catalyst principles. A considerable challenge however is the fact that chiral catalyst derivatives occasionally show lower reactivities than achiral ones, making identification of a suited chiral catalyst potentially difficult. Within this project we wish to establish well-known isothioureas as catalysts for reactions of allenoates, a strategy that has so far not been reported. We are confident that this strategy will allow for orthogonal reactivities as compared to established allenoate transformations, thus providing novel strategies for allenoate-based cyclizations.
Project Publications:
Please see publications 106 and 110 from our general publication overview for a first proof-of-concept for the feasibility of the methods developed within this project.
Asymmetric synergistic ammonium (hypo)iodite-imine catalysis
(LIT seed project LIT-2019-8-SEE-111, 11/2020 - 12/2022)
The introduction of novel catalytic (asymmetric) strategies and concepts is a task of high relevance and recently we found that the use of H2O2 in combination with simple imines in the presence of a chiral ammonium iodide catalyst allows for promising selectivities in the asymmetric a-hydroxylation of b-ketoesters. Very interestingly, this oxidation reaction requires the synergistic combination of the ammonium iodide catalyst and a simple catalytically active imine to proceed with high yields and selectivities, thus resulting in a unique catalysis principle. The high selectivities obtained with this unique cooperative catalysis principle prompted us to explore this concept further within the context of this project.
Project Publications:
Please see publications 77, 88, 92, 101 and 103 from our general publication overview for details about the research carried out in the course of this project.
Syntheses of Chiral Fluorinated Amino Acids and Peptides
(FWF stand alone project P30237, 01/2018 - 12/2021)
Abstract: The replacement of hydrogen atoms by fluorine in organic molecules has become a very important and powerful tool in various fields of research, mainly because of the fact that such a modification usually has a very dramatic effect on the physical, chemical, biological, and medical properties of these compounds. One class of fluorine containing compounds that has attracted a lot of interest for biomedical applications are fluorine containing amino acids and peptides. However, it is fair to say that the synthesis of chiral fluorinated amino acids or peptides still represents a major synthetic challenge. In this project plan to overcome this current limitation by establishing novel catalytic synthesis strategies to access chiral fluorinated a- and b-amino acid derivatives or peptides. Once successful, this project should thus deliver new powerful synthesis tools that will be of interest to organic and medicinal chemists and will finally give access to compounds that were not accessible with the existing state of the art methods.
Project Publications:
Please see publications 63, 67, 68, 70, 71, 72, 73, 76, 80, 81, 82, 86 and 90 from our general publication overview for details about the research carried out in the course of this project.
Syntheses of (Chiral) Hetero- and Carbocycles Using Ammonium Enolates
(FWF stand alone project P26387, 09/2014 - 10/2018)
Abstract: The development of powerful, generally applicable, and highly efficient synthetic transformations is one of the ultimate goals in (organic) chemistry, especially considering the increasing demand for complex molecules with a variety of different functions in our society nowadays (pharmaceutical applications, agrochemicals, material science,..). Amongst the frequently found structural motives, highly functionalized small and medium ring size chiral (hetero)-cycles are of uttermost importance, as they present the key-scaffold in a large variety of biologically active (natural) products. In addition they serve as versatile intermediates in the synthesis of biologically active molecules. Accordingly, novel powerful strategies to access them in an efficient, economic, and direct fashion are an important task, not only for organic chemists working academia, but also for medicinal chemists searching for new lead compounds or for industrial (pharmaceutical) applications.
The use of (chiral) ammonium enolates (either preformed or generated in situ by using a catalyst) to carry out demanding stereoselective reactions is a powerful and unique strategy, giving access to transformations that are not possible using any other (catalytic) methods. However, having a closer look at the use of this unique strategy to facilitate (stereoselective) organic reactions it is fair to say that, although impressive (stereoselective) examples have been reported recently, it seems reasonable that this methodology holds much more promise for further investigations and the development of significantly more complex and outstanding applications. Thus, it is the main target of this project to address transformations that will give access to a variety of highly important cyclic structural motives that represent major synthesis challenges, as other commonly employed methods are still not powerful enough.
Accordingly, this project will introduce versatile strategies to facilitate the activation and use of easily available starting materials in a unique and unprecedented fashion and thus will provide new powerful methodologies to the standard toolbox employed by chemists to obtain important highly functionalized cyclic compounds straightforwardly.
Project Publications:
Please see publications 31, 33, 35, 39, 40, 44, 47, 48, 49, 52, 53, 54, 56, 58, 61, 66 from our general publication overview for further details about the research carried out in the course of this project.
Chiral Ammonium Salts Meet Transition Metal Catalysis
(FWF Lise Meitner funding M1602, 02/2014 - 02/2015; PI: Dr Raghunath Chowdhury)
Abstract: The efficient and stereoselective construction of complex molecular architectures is one of the ultimate goals in chemistry and the use of small molecule organic catalysts (organocatalysts) has proven to be highly useful to achieve complex transformations starting from simple easily available starting materials in a large variety of different case studies. Hereby, non-covalent catalysis modes are amongst the most versatile approaches to mimic Nature, usually benefitting from mild and easy to handle reaction conditions, environmentally benign reagents, and high efficiencies. The use of chiral cations (especially chiral ammonium salt phase-transfer catalysts (PTCs)) as catalysts has obtained a prominent and outstanding position and has contributed significantly to the field of asymmetric catalysis. The enormous potential of these catalysts lies in the fact that (theoretically) every reaction involving anionic or even just highly Lewis basic intermediates (starting materials) can be affected and controlled by these cationic catalysts.
Considering the high potential of chiral PTCs to facilitate reactions where other methods clearly fail, we are confident that the use of chiral PTCs holds much promise to facilitate a series of highly demanding complex transformations, thus addressing some longstanding synthesis challenges. Although the synergistic combination of complementary activation modes has emerged as a powerful tool for complex transformations, it comes as a surprise that so far only a few reports about the synergistic combination of chiral PTCs with complementary activation modes have been published.
It is the main target of this project to carry out a systematic feasibility study of the combined use of chiral phase-transfer catalysts with complementary transition metal catalysts in a synergistic (or cascade) fashion. The high value of such an approach will be the benefit of employing the unique nucleophile-activation potential of chiral cation-based catalysts in combination with the well-described electrophile activating properties of transition metal catalysts. Accordingly, the successful implementation of the herein investigated reactions will broaden the field of asymmetric catalysis in general, as substrates which have so far not been useable in such approaches in a catalytic stereoselective manner can be directly employed to obtain valuable chiral compounds in just a single step. Thus this project should provide the community with outstanding new tools to solve longstanding problems and to get access to chiral building blocks that are otherwise only difficultly accessible in a stereoselective fashion under mild and environmentally friendly conditions by using easily tuneable and easy to handle catalysts.
Project Publications:
Please see publications 32 and 50 from our general publication overview for further details about the research carried out in the course of this project.
Novel Tartaric Acid Derived Asymmetric Organocatalysts
(FWF stand alone project P22508, 09/2010 - 09/2014)
Abstract: The ability to control the three-dimensional structure of the molecular architecture is one of the primary targets in synthetic organic chemistry. Amongst the various ways of creating enantiomerically enriched products, catalytic methods are considered to be the most appealing as the use of stoichiometric amounts of valuable chiral reagents can be avoided, thus making optimum use of the chiral pool. Besides enzymatic and metal catalyzed asymmetric transformations, the use of sub-stoichiometric amounts of organic molecules (so called organocatalysts) has proven to possess an enormous potential for the catalysis of stereoselective reactions. Among the easily available natural chiral sources, tartaric acid has obtained a prominent position not only for historical reasons, but especially due to the fact that both enantiomers are readily available from natural sources. Surprisingly, although tartaric acid derivatives are almost omnipresent in metal catalysis, their use as chiral organocatalysts has so far been limited to a few applications only. Due to the fact that tartaric acid represents a very unique carbon skeleton possessing electronic and steric properties different from other commonly used chiral moieties the primary target of this project is the synthesis of novel tartaric acid derived organocatalysts and their application in asymmetric catalysis. As both, L- and D-tartaric acid are easily and cheaply available, access to both enantiomers of new catalysts is guaranteed.
Among the different activation modes, which are hitherto known in organocatalysis, the main focus herein lies on the synthesis of chiral ammonium salts, chiral Lewis acids/bases, and bifunctional catalysts. These compounds should enable the catalysis of a wide variety of different fundamental reactions. Therefore, in each case the ability of the new catalysts for the asymmetric catalysis of reactions like alkylations, allylations, aldol type reactions, or cyclizations will be investigated. Furthermore, a careful investigation concerning the crucial structural parameters of the novel catalysts will be undertaken.
The development of these novel organocatalysts will broaden the scope of organocatalysis as it will make use of one of the most easily available and cheapest natural chiral sources which has so far not been exploited much in this field. Furthermore, due to the characteristic and unique structural features of these catalysts, different reactivities compared to the currently used organocatalysts seem to be possible.
Project Publications:
Please see publications 13, 15, 18, 21, 22, 23, 25, 26, 27, 29 from our general publication overview for further details about the research carried out in the course of this project.
Please check our publication list for further details on all our fruitful collaborations with the following groups:
Mauro Adamo, opens an external URL in a new window, Royal College of Surgeons in Ireland (RCSI); Ireland
Jean-François Brière, opens an external URL in a new window, Laboratoire COBRA, Université de Rouen Normandie, France
David Díaz Díaz, opens an external URL in a new window, University of Regensburg, Germany
Steven Kass, opens an external URL in a new window, University of Minnesota, US
Antonio Massa, opens an external URL in a new window, University of Salerno, Italy
Armin Ofial, opens an external URL in a new window, LMU Munich, Germany
Jiří Pospíšil, opens an external URL in a new window, Palacky University Olomouc, Czech Republic
Raphaël Robiette, opens an external URL in a new window, Université catholique de Louvain, Belgium
Andrew. D. Smith, opens an external URL in a new window, University of St Andrews, UK
Mathew Vetticatt, opens an external URL in a new window, University of Binghamton, US
<2024>
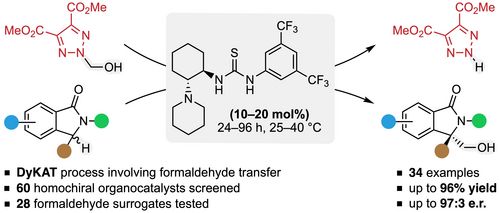
111. "Asymmetric Organocatalyzed Transfer Hydroxymethylation of Isoindolinones Using Formaldehyde Surrogates"
David Svestka, Pavel Bobal,* Mario Waser, and Jan Otevrel*
Org. Lett., 2024, 26, 2505–2510. [full text], opens an external URL in a new window
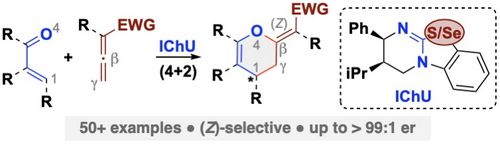
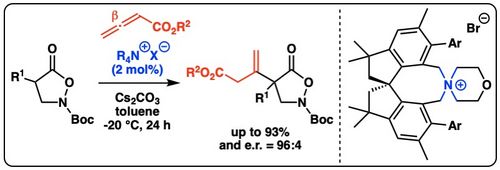
110. "Enantioselective Syntheses of 3,4-Dihydropyrans Employing Isochalcogenourea-Catalyzed Formal (4+2)-Cycloadditions of Allenoates"
Magdalena Piringer, Mario Hofer, Lukas S. Vogl, Peter Mayer, and Mario Waser*
Adv. Synth. Catal., 2024, accepted for publication. [full text], opens an external URL in a new window
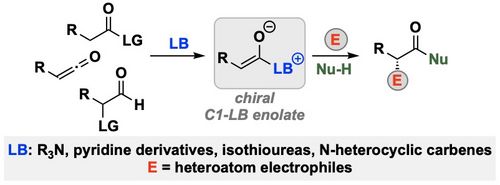
109. "Enantioselective α-Heterofunctionalization Reactions of Catalytically Generated C1-Lewis Base Enolates"
Magdalena Piringer, Lotte Stockhammer, Lukas Vogl, David Weinzierl, Paul Zebrowski and Mario Waser*
Tetrahedron Chem, 2024, 9, 100063. [full text], opens an external URL in a new window
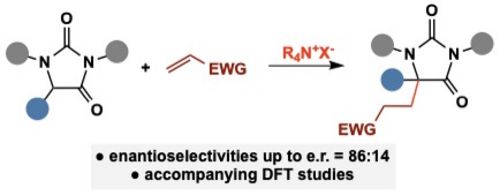
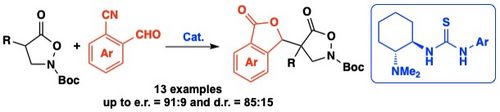
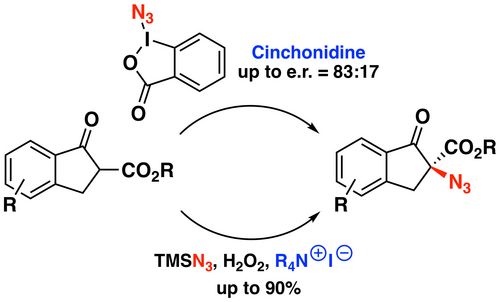
108. "Chiral Quaternary Ammonium Salt-Catalyzed Enantioselective Addition Reactions of Hydantoins"
Katharina Röser, Lucas Prameshuber, Sajid Jahangir, Sharath C. Mallojjala, Jennifer S. Hirschi,* and Mario Waser*
Helv. Chim. Acta, 2024, 107, e202300234. [full text], opens an external URL in a new window
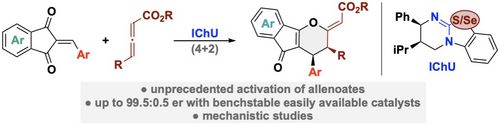
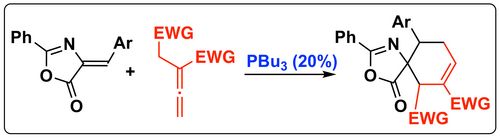
107. "Chiral Isochalcogenourea-Catalysed Enantioselective (4+2) Cycloadditions of Allenoates"
Lukas S. Vogl, Peter Mayer, Raphaël Robiette,* and Mario Waser*
Angew. Chem. Int. Ed, 2024, 63, e202315345. [full text], opens an external URL in a new window
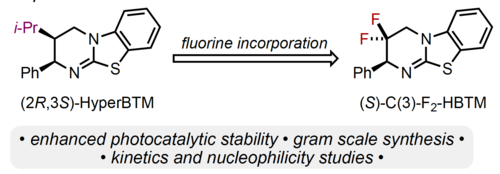
106. "Unveiling the impact of a CF2 motif in the isothiourea catalyst skeleton: Evaluating C(3)-F2-HBTM and its catalytic activity"
Matthew T. Westwood, Kevin Kasten, Lotte Stockhammer, Roberto del Río-Rodríguez, Jose A. Fernández-Salas, Andreas Eitzinger, Alexandra M. Z. Slawin, Mario Waser, Jose Alémán, Armin R. Ofial,* Andrew D. Smith*
Arkivoc, 2024, 4, 202312093. [full text], opens an external URL in a new window
<2023>
105. "Asymmetric syntheses of valuable chiral trifluoromethylated enamides"
David Weinzierl and Mario Waser*
Chem Catal., 2023, 3, 100829. [full text], opens an external URL in a new window
(Invited preview for Prof. Belén Martín-Matute's contribution in the same issue)
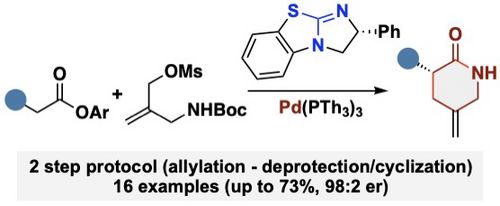
104. "Cooperative Chiral Lewis Base/Palladium-Catalyzed Asymmetric Syntheses of Methylene-Containing d-Lactams"
Paul Zebrowski, Uwe Monkowius, and Mario Waser*
Eur. J. Org. Chem., 2023, 26, e202300982. [full text], opens an external URL in a new window
(Highlighted in Organic Chemistry Portal, opens an external URL in a new window)
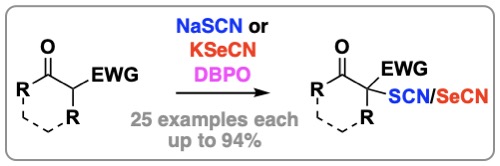
103. "Dibenzoylperoxide-Mediated Oxidative α-Thio/Seleno-Cyanation of β-Ketoesters and Oxindoles"
Christopher Mairhofer, Katharina Röser, Meysam Aryafard, Markus Himmelsbach, and Mario Waser*
Eur. J. Org. Chem., 2023, 26, e202300969. [full text], opens an external URL in a new window
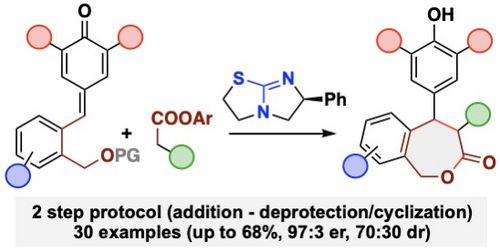
102. "Chiral Lewis Base-Catalysed Asymmetric Syntheses of Benzo-fused ε-Lactones"
Lotte Stockhammer, Maximilian Radetzky, Syeda Sadia Khatoon, Matthias Bechmann, and Mario Waser*
Eur. J. Org. Chem., 2023, 26, e202300704. [full text], opens an external URL in a new window
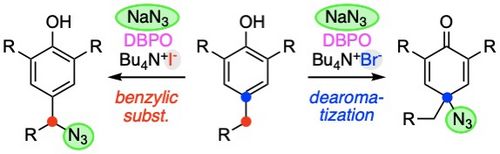
101. "Dibenzoylperoxide as a Versatile Oxidant for Oxidative Azidations of Phenols Using Quaternary Ammonium Iodides or Bromides"
Christopher Mairhofer, Mario Waser*
Adv. Synth. Catal., 2023, 365, 2757-2762. [full text], opens an external URL in a new window
100. "Photochemical Wolff Rearrangement Initiated Generation and Subsequent α-Chlorination of C1 Ammonium Enolates"
David Weinzierl, Magdalena Piringer, Paul Zebrowski, Lotte Stockhammer, Mario Waser*
Org. Lett., 2023, 25, 3126-3130. [full text], opens an external URL in a new window
(Highlighted in Organic Chemistry Portal, opens an external URL in a new window)
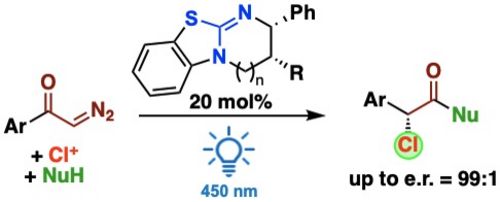
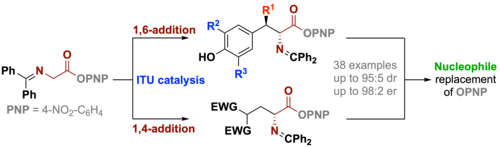
99. "Isothiourea-Catalyzed Enantioselective Functionalisation of Glycine Schiff Base Aryl Esters via 1,6- and 1,4-additions"
Lotte Stockhammer, Rebecca Craik, Uwe Monkowius, David B. Cordes, Andrew D. Smith,* Mario Waser*
ChemistryEurope, 2023, 1, e202300015. [full text], opens an external URL in a new window
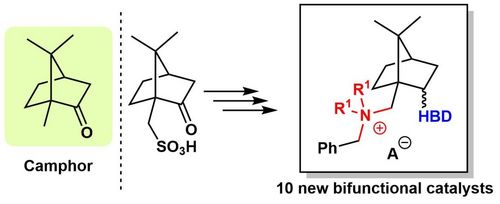
98. "Synthesis and Catalytic Activity of Bifunctional Phase-Transfer Organocatalysts Based on Camphor"
Luka Ciber, Franc Požgan, Helena Brodnik, Bogdan Štefane, Jurij Svete, Mario Waser*, and Uroš Grošelj*
Molecules, 2023, 28, 1515. [full text], opens an external URL in a new window
97. "Enantioselective β-selective addition of isoxazolidin-5-ones to allenoates catalyzed by quaternary ammonium salts"
Paul Zebrowski, Katharina Röser, Daniel Chrenko, Jiri Pospisil and Mario Waser*
Synthesis, 2023, 55, 1706-1713. [full text], opens an external URL in a new window
(Invited contribution to a special issue dedicated to Prof. Cristina Nevado)
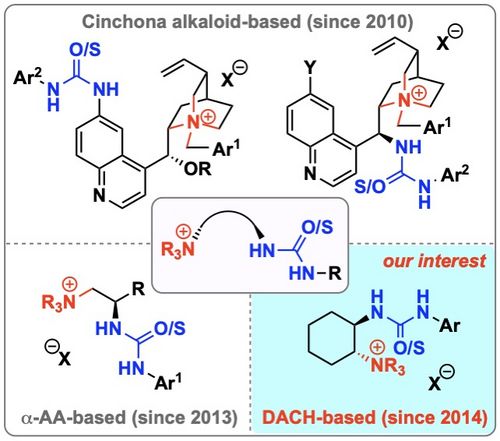
96. "(Thio)urea containing chiral ammonium salt catalysts"
Mario Waser,* Michael Winter, and Christopher Mairhofer
Chem. Rec., 2023, 23, e202200198. [full text], opens an external URL in a new window
(Invited contribution to a special issue on asymmetric organocatalysis)
95. "Recent Developments and Trends in Asymmetric Organocatalysis"
Olga Garcia Mancheño* and Mario Waser*
Eur. J. Org. Chem., 2023, 26, e202200950. [full text], opens an external URL in a new window
(Invited perspective article for a special online collection on asymmetric organocatalysis)
<2022>
94. "Stereoselective syntheses of masked b-amino acid containing phthalides"
Lorenzo Serusi, Paul Zebrowski, Johannes Schörgenhumer, Antonio Massa, and Mario Waser*
Helv. Chim. Acta, 2022, 105, e202200110. [full text], opens an external URL in a new window
(Invited contribution to a special issue dedicated to Prof. Janine Cossy)
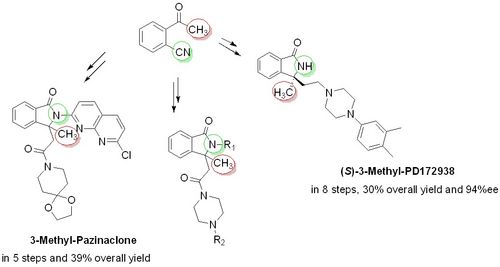
93. "(Scalable (enantioselective) syntheses of novel 3-methylated analogs of Pazinaclone, (S)-PD172938 and related biologically relevant isoindolinones"
Antonia Di Mola,* Giorgia Nicastro, Lorenzo Serusi, Rosanna Filosa, Mario Waser, and Antonio Massa*
Molecules, 2022, 27, 5647. [full text], opens an external URL in a new window
92. "Oxidative decarboxylative ammonium hypoiodite-catalysed dihydrobenzofuran synthesis"
Katharina Röser, Anna Scheucher, Christopher Mairhofer, Matthias Bechmann, and Mario Waser*
Org. Biomol. Chem., 2022, 20, 3273–3276. [full text], opens an external URL in a new window
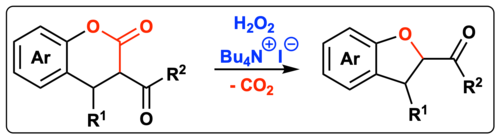
91. "Catalytic Enantioselective Decarboxylative Aldol Reactions of Malonic Acid Half Thio(oxy)ester and β-Ketoacids"
Raghunath Chowdhury,* Akhil K. Dubey, and Mario Waser
Eur. J. Org. Chem., 2022, e202200146. [full text], opens an external URL in a new window
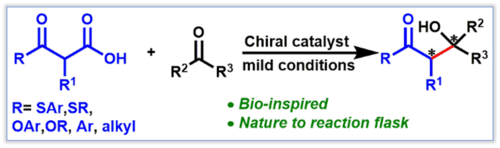
90. "Enantioselective organocatalytic syntheses of α-selenated α- and β-amino acid derivatives"
Victoria Haider, Paul Zebrowski, Jessica Michalke, Uwe Monkowius, and Mario Waser*
Org. Biomol. Chem., 2022, 20, 824-830. [full text], opens an external URL in a new window
(Highlighted in Synfacts, opens an external URL in a new window)
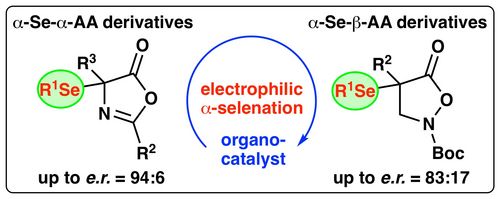
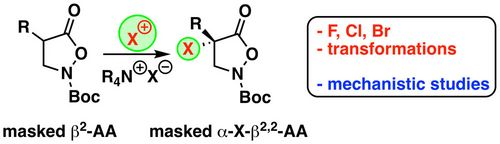
89. "Enantioselective catalytic synthesis of α-halogenated-α-aryl-β2,2-amino acid derivatives"
Paul Zebrowski, Isabella Eder, Andreas Eitzinger, Sharath Chandra Mallojjala,* and Mario Waser*
ACS Org. Inorg. Au, 2022, 2, 34-43. [full text], opens an external URL in a new window
(Highlighted in Synfacts, opens an external URL in a new window)
<2021>
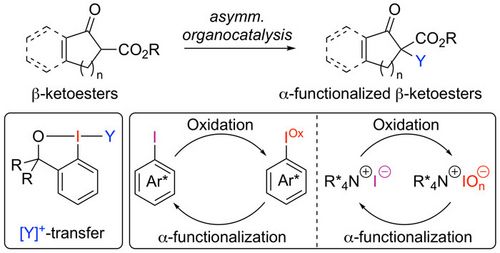
88. "Organocatalytic asymmetric α-functionalizations of β-ketoesters with hypervalent iodine-based reagents and catalysts"
Christopher Mairhofer, Lotte Stockhammer, and Mario Waser*
Arkivoc, 2021, vii, 112-127. [full text], opens an external URL in a new window
87. "Enantioselective α-Chlorination Reactions of in situ Generated C1 Ammonium Enolates under Base-Free Conditions"
Lotte Stockhammer, David Weinzierl, Thomas Bögl, and Mario Waser*
Org. Lett., 2021, 23, 6143−6147. [full text], opens an external URL in a new window
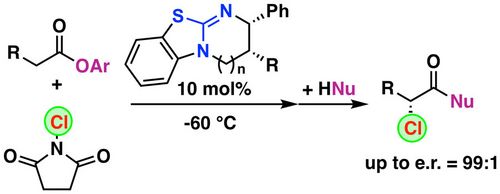
86. "Enantioselective synthesis of acyclic orthogonally functionalized compounds bearing a quaternary stereocenter using chiral ammonium salt catalysis"
Katharina Röser, Bettina Berger, Michael Widhalm,* and Mario Waser*
ChemistryOpen, 2021, 10, 756-759. [full text], opens an external URL in a new window
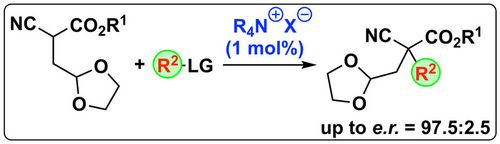
85. "Synthesis of [2.2]paracyclophane-based glycidic amides using chiral ammonium ylides"
David Weinzierl and Mario Waser*
Helv. Chim. Acta, 2021, 104, e2100073. [full text], opens an external URL in a new window
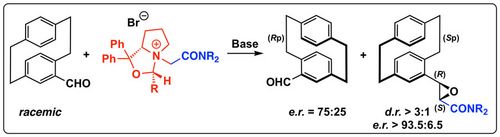
84. "Study of ground state interactions of enantiopure chiral quaternary ammonium salts and amides, nitroalkanes, nitroalkenes, esters, heterocycles, ketones and fluoroamides"
Grazia Bencivenni, Diana Salazar Illera, Maria Moccia, Kendall N. Houk, Joseph A. Izzo, Johanna Novacek, Paolo Grieco, Mathew J. Vetticatt,* Mario Waser,* Mauro F. A. Adamo*
Chem. Eur. J., 2021, 27, 11352-11366. [full text], opens an external URL in a new window
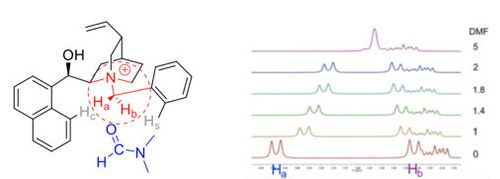
83. "Chiral Isothiourea-Catalyzed Kinetic Resolution of 4-Hydroxy[2.2]paracyclophane"
David Weinzierl and Mario Waser*
Beilstein J. Org. Chem., 2021, 17, 800-804. [full text], opens an external URL in a new window
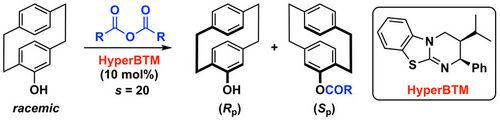
82. "Enantioselective Bifunctional Ammonium Salt-Catalyzed Syntheses of 3-CF3S-, 3-RS-, and 3-F-Substituted Isoindolinones"
Andreas Eitzinger, Jan Otevrel, Victoria Haider, Antonio Macchia, Antonio Massa, Kirill Faust, Bernhard Spingler, Albrecht Berkessel, and Mario Waser*
Adv. Synth. Catal., 2021, 363, 1955-1962. [full text], opens an external URL in a new window
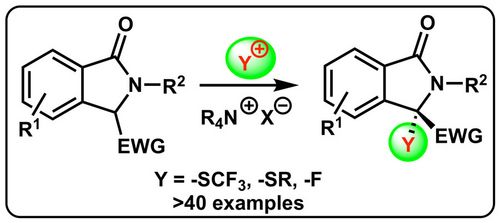
81. "Enantiospecific deoxyfluorination of cyclic α-OH-β-ketoesters"
Christopher Mairhofer, Victoria Haider, Thomas Bögl, and Mario Waser*
Org. Biomol. Chem., 2021, 19, 162-165. [full text], opens an external URL in a new window
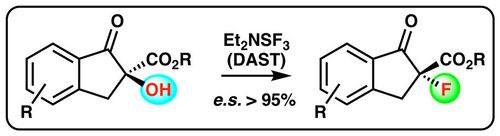
80. "Recent Progress in the Asymmetric Syntheses of α-Heterofunctionalized (Masked) α- and β-Amino Acid Derivatives"
Isabella Eder, Victoria Haider, Paul Zebrowski, and Mario Waser*
Eur. J. Org. Chem., 2021, 202-219. [full text], opens an external URL in a new window
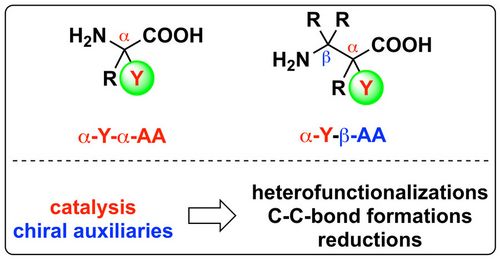
79. "Asymmetric α-chlorination of β-ketoesters using hypervalent iodine-based Cl-transfer reagents in combination with Cinchona alkaloid catalysts"
Lotte Stockhammer, Johannes Schörgenhumer, Christopher Mairhofer, and Mario Waser*
Eur. J. Org. Chem., 2021, 82-86. [full text], opens an external URL in a new window
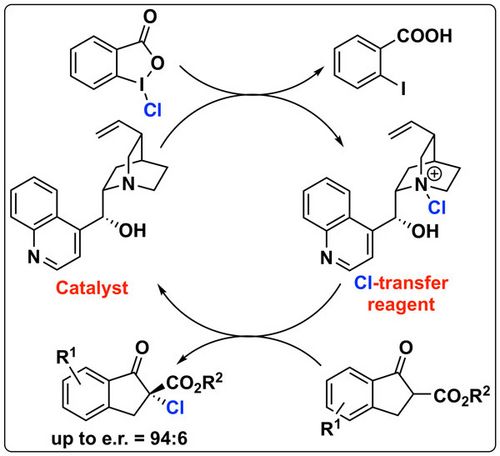
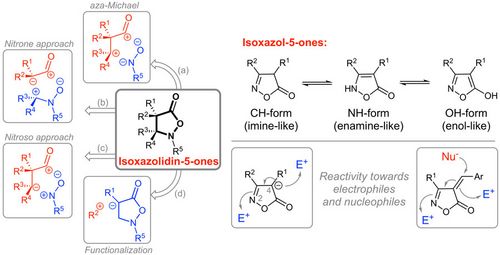
78. "Asymmetric synthesis of Isoxazol-5-ones and Isoxazolidin-5-ones"
Antonio Macchia, Andreas Eitzinger, Jean-François Brière,* Mario Waser,* and Antonio Massa*
Synthesis 2021, 53, 107-122. [full text], opens an external URL in a new window
<2020>
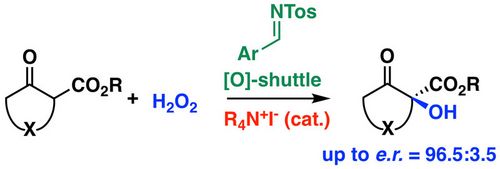
77. "Synergistic Ammonium (Hypo)-Iodite/Imine Catalysis for the Asymmetric α-Hydroxylation of β-Ketoester"
Christopher Mairhofer, Johanna Novacek, Mario Waser*
Org. Lett., 2020, 22, 6138-6142. [full text], opens an external URL in a new window
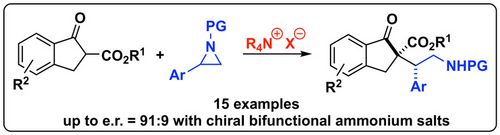
76. "Ammonium Salt-Catalyzed Ring-Opening of Aryl-Aziridines with β-Ketoesters"
Victoria Haider, Viktoria Kreuzer, Maximilian Tiffner, Bernhard Spingler, Mario Waser*
Eur. J. Org. Chem., 2020, 5173-5177. [full text], opens an external URL in a new window
75. "Trisubstituted highly activated benzo[d]thiazol-2-yl-sulfone-containing olefins as building blocks in organic synthesis"
Ondřej Kováč, František Zálešák, David Bon, Lukas Roiser, Lubomír Baar, Mario Waser, Jiri Pospisil*
J. Org. Chem., 2020, 85, 7192-7206. [full text], opens an external URL in a new window
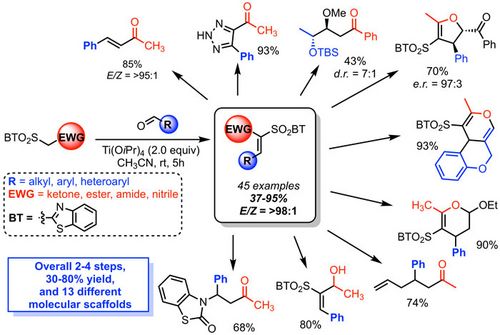
74. "Chiral Phase Transfer Catalysis in the Asymmetric Synthesis of a 3,3-Disubstituted Isoindolinone and Determination of Its Absolute Configuration by VCD Spectroscopy"
Guglielmo Monaco, Maximilian Tiffner, Antonia Di Mola, Wouter Herrebout, Mario Waser, Antonio Massa*
Molecules, 2020, 25, 2272. [full text, opens an external URL in a new window]
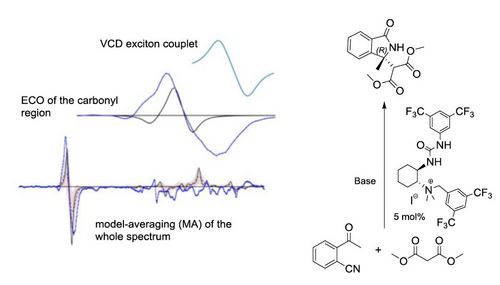
73. "CF3-Containing para-Quinone Methides for Organic Syntheses"
Michael Winter, Roman Schütz, Andreas Eitzinger, Armin R. Ofial, Mario Waser*
Eur. J. Org. Chem., 2020, 3812-3817. [full text], opens an external URL in a new window
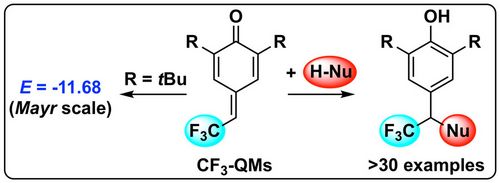
72. "Electrophilic Reactivities of Vinyl p-Quinone Methides"
Andreas Eitzinger, Robert. J. Mayer, Nathalie Hampel, Peter Mayer, Mario Waser, Armin R. Ofial*
Org. Lett., 2020, 22, 2182-2186. [full text], opens an external URL in a new window
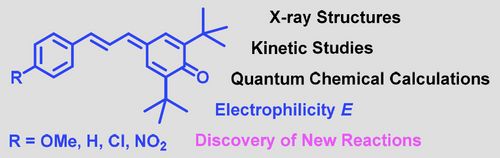
71. "Enantioselective Catalytic Synthesis of a-Aryl-a-SCF3-b2,2-Amino Acids"
Andreas Eitzinger, Jean-François Brière, Dominique Cahard,* Mario Waser*
Org. Biomol. Chem., 2020, 18, 405-408. [full text], opens an external URL in a new window
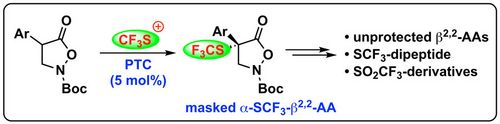
70. "Quaternary b2,2-Amino Acid Derivatives by Asymmetric Addition of Isoxazolidin-5-ones to para-Quinone Methides"
Andreas Eitzinger, Michael Winter, Johannes Schörgenhumer, Mario Waser*
Chem. Commun, 2020, 56, 579-582. [full text], opens an external URL in a new window
(Highlighted in Synfacts, opens an external URL in a new window)

69. "A flexible strategy for the synthesis of bifunctional 6’-(thio)-urea containing Cinchona alkaloid ammonium salts"
Johannes Schörgenhumer, Stefan Otte, Victoria Haider, Johanna Novacek, Mario Waser*
Tetrahedron, 2020, 76, 130816. [full text], opens an external URL in a new window
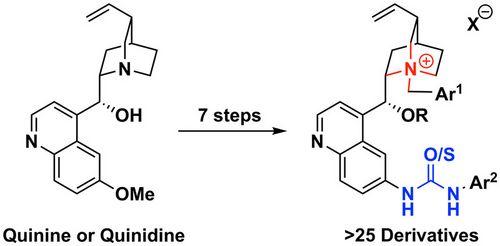
<2019>
68. "Pd-catalyzed allylation of imines to access a-CF3-substituted a-amino acid derivatives"
Michael Winter, Hyunwoo Kim, Mario Waser*
Eur. J. Org. Chem., 2019, 7122-7127. [full text], opens an external URL in a new window
(Highlighted in Synfacts, opens an external URL in a new window)
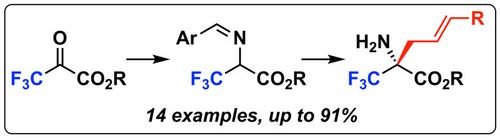
67. "Synthesis of a-CF3-proline derivatives by means of a formal (3+2)-cyclisation between trifluoropyruvate imines and Michael acceptors"
Michael Winter, Kirill Faust, Markus Himmelsbach, Mario Waser*
Org. Biomol. Chem., 2019, 17, 5731-5735. [full text], opens an external URL in a new window
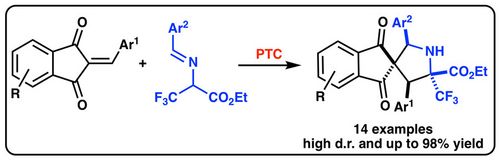
66. "Enantioselective Catalytic (4+1)-Cyclization of ortho-Hydroxy-para-Quinone Methides with Allenoates"
Katharina Zielke, Ondřej Kováč, Michael Winter, Jiří Pospíšil, Mario Waser*
Chem. Eur. J., 2019, 25, 8163-8168. [full text], opens an external URL in a new window
(Highlighted in Synfacts, opens an external URL in a new window)
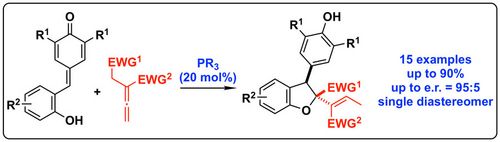
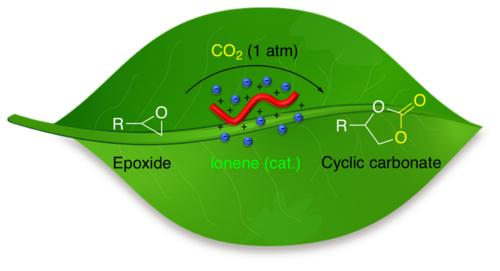
65. "(Thio)urea Containing Quaternary Ammonium Salts for the CO2-Fixation with Epoxides"
Johannes Schörgenhumer, Maximilian Tiffner, and Mario Waser*
Monatsh. Chem., 2019, 150, 789-794. [full text], opens an external URL in a new window
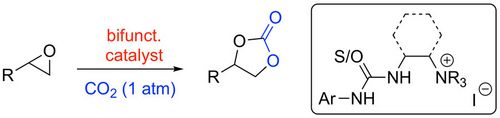
64. "Synthesis and Organocatalytic Asymmetric Nitro-aldol Initiated Cascade Reactions of 2-Acylbenzonitriles Leading to 3,3-Disubstituted Isoindolinones"
Fabio Romano, Antonia Di Mola, Laura Palombi, Maximilian Tiffner, Mario Waser,* and Antonio Massa*
Catalysts, 2019, 9, 327. [full text], opens an external URL in a new window
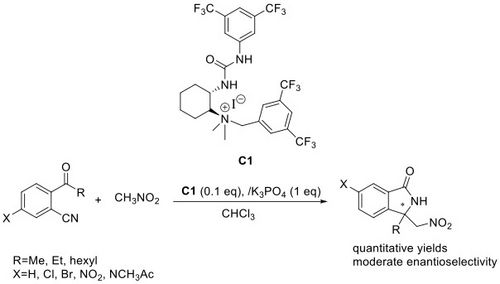
<2018>
63. "Asymmetric phase-transfer catalysed b-addition of isoxazolidin-5-ones to MBH carbonates"
Vito Capaccio, Katharina Zielke, Andreas Eitzinger, Antonio Massa, Laura Palombi, Kirill Faust, and Mario Waser*
Org. Chem. Front., 2018, 5, 3336-3340. [full text], opens an external URL in a new window
62. "Transition Metal-Free Coupling of Terminal Alkynes and Hypervalent Iodine-Based Alkyne-Transfer Reagents to Access Unsymmetrical 1,3-Diynes"
Johannes Schörgenhumer and Mario Waser*
Org. Biomol. Chem., 2018, 16, 7561-7563. [full text], opens an external URL in a new window
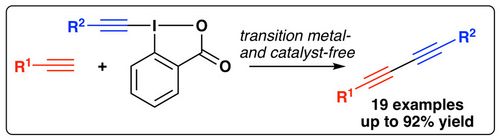
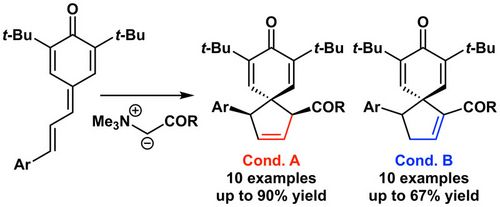
61. "Formal (4+1)-Cyclization of Ammonium Ylides with Vinylogous para-Quinone Methides"
Lukas Roiser, Katharina Zielke, and Mario Waser*
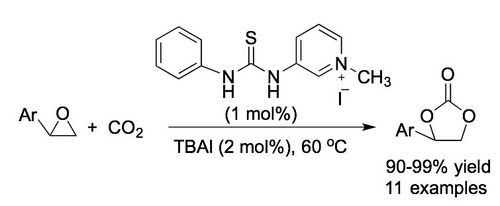
Synthesis, 2018, 50, 4047-4054. [full text], opens an external URL in a new window
60. "Synthesis of Cyclic Organic Carbonates Using Atmospheric Pressure CO2 and Charge-Containing Thiourea Catalysts"
Yang Fan, Maximilian Tiffner, Johannes Schörgenhumer, Raphaël Robiette, Mario Waser* and Steven R. Kass*
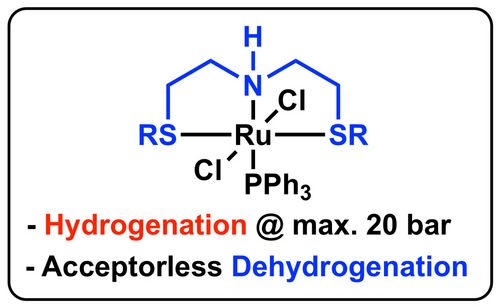
J. Org. Chem., 2018, 83, 9991−10000. [full text], opens an external URL in a new window
59. "SNS-Ligands for Ru-Catalyzed Homogeneous Hydrogenation and Dehydrogenation Reactions"
Johannes Schörgenhumer, Axel Zimmermann,* and Mario Waser*
Org. Process Res. Dev., 2018, 22, 862-870. [full text], opens an external URL in a new window
58. "Syntheses of Highly-Functionalized Spirocyclohexenes by Formal (4+2)-Annulation of Arylidene Azlactones with Allenoates "
Andreas Eitzinger, Katharina Zielke, Michael Widhalm, Raphaël Robiette,* and Mario Waser*
Asian J. Org. Chem., 2018, 7, 1620-1625. [full text], opens an external URL in a new window
57. "Cationic Polymers Bearing Quaternary Ammonium Groups-Catalyzed CO2 Fixation with Epoxides"
Maximilian Tiffner, Marleen Häring, David Díaz Díaz*, and Mario Waser*
Top. Catal., 2018, 61, 1545-1550. [full text], opens an external URL in a new window
56. "Ammonium Ylide-Mediated Cyclizations"
Lukas Roiser, Katharina Zielke, and Mario Waser*
Asian J. Org. Chem., 2018, 7, 852-864. [full text], opens an external URL in a new window
55. "Towards an Asymmetric Organocatalytic α-Azidation of β-Ketoesters"
Maximilian Tiffner, Lotte Stockhammer, Johannes Schörgenhumer, Katharina Röser, and Mario Waser*
Molecules, 2018, 23, 1142-1150. [full text], opens an external URL in a new window
54. "Formal (4+1)-Addition of Allenoates to ortho-Quinone Methides"
Katharina Zielke and Mario Waser*
Org. Lett., 2018, 20, 768-771. [full text], opens an external URL in a new window
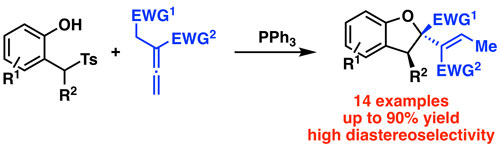
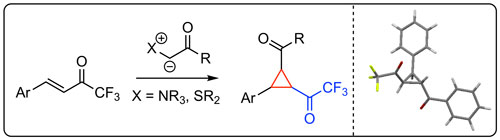
53. "Synthesis of Trifluoroacetyl-Substituted Cyclopropanes Using Onium Ylides"
Michael Winter, Christina Gaunersdorfer, Lukas Roiser, Katharina Zielke, Uwe Monkowius, and Mario Waser*
Eur. J. Org. Chem., 2018, 418-421. [full text], opens an external URL in a new window
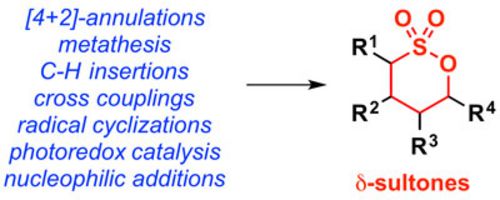
52. "Progress in the synthesis of δ-sultones"
Christina Gaunersdorfer and Mario Waser*
Monatsh. Chem., 2018, 149, 701-714. [full text], opens an external URL in a new window
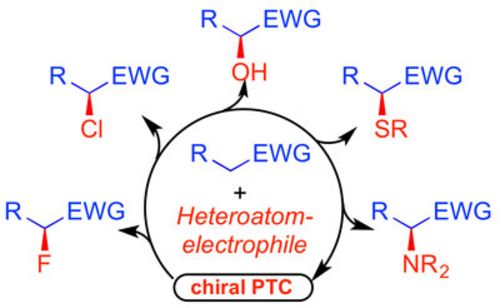
<2017>
51. "Chiral Phase-Transfer Catalysis in the Asymmetric a-Heterofunctionalization of Prochiral Nucleophiles"
Johannes Schörgenhumer, Maximilian Tiffner, and Mario Waser*
Beilstein J. Org. Chem., 2017, 13, 1753-1769. [full text], opens an external URL in a new window
50. "Hypervalent Iodine-Mediated a-Arylation of Glycine Schiff Base"
Johannes Schörgenhumer, Raghunath Chowdhury,* and Mario Waser*
Chem. Data Collect., 2017, 11-12, 36-39. [full text], opens an external URL in a new window
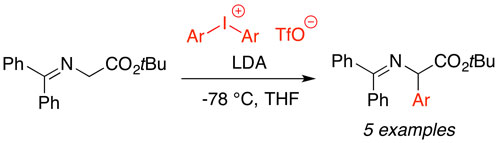
49. "Enantioselective spirocyclopropanation of para-quinone methides using ammonium ylides"
Lukas Roiser and Mario Waser*
Org. Lett., 2017, 19, 2338-2341. [full text], opens an external URL in a new window
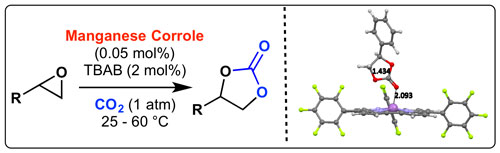
48. "CO2 Fixation with Epoxides under Mild Conditions with a Cooperative Metal Corrole - Quaternary Ammonium Salt Catalyst System"
Maximilian Tiffner, Sabrina Gonglach, Michael Haas, Wolfgang Schöfberger,* and Mario Waser*
Chem. Asian J., 2017, 12, 1048-1051. [full text], opens an external URL in a new window
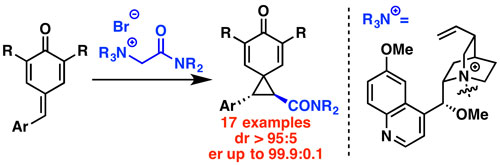
47. "Asymmetric synthesis of 2,3-dihydrobenzofurans via a (4+1) annulation between ammonium ylides and in situ generated ortho-quinone methides"
Nicole Meisinger, Lukas Roiser, Uwe Monkowius, Markus Himmelsbach, Raphaël Robiette,* and Mario Waser*
Chem. Eur. J., 2017, 23, 5137-5142. [full text], opens an external URL in a new window
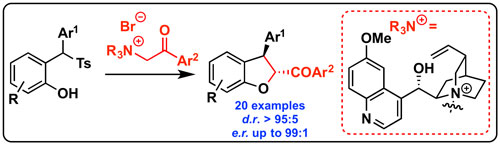
46. "On-Surface Site-Selective Cyclization of Corrole Radicals"
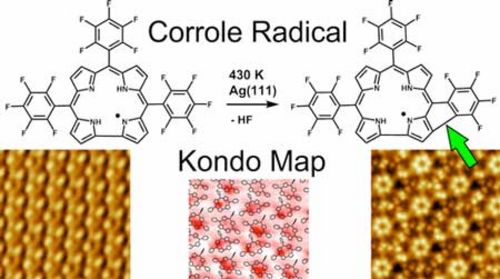
Stefano Tebi, Mateusz Paszkiewicz, Hazem Aldahhak, Francesco Allegretti, Sabrina Gonglach, Michael Haas, Mario Waser, Peter S. Deimel, Pablo Casado Aguilar, Yi-Qi Zhang, Anthoula C. Papageorgiou, David A. Duncan, Johannes V. Barth, Wolf G. Schmidt, Reinhold Koch, Uwe Gerstmann,* Eva Rauls,* Florian Klappenberger,* Wolfgang Schöfberger* and Stefan Müllegger*
ACS Nano, 2017, 11, 3383-3391. [full text], opens an external URL in a new window
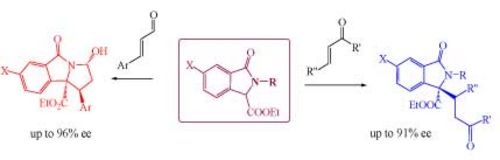
45. "A systematic study on the use of different organocatalytic activation modes for asymmetric conjugated addition reactions of isoindolinones"
Francesco Scorzelli, Antonia Di Mola, Francesco De Piano, Consiglia Tedesco, Laura Palombi, Rosanna Filosa, Mario Waser and Antonio Massa*
Tetrahedron, 2017, 73, 819-828. [full text], opens an external URL in a new window
44. "On the origin of the stereoselectivity in chiral amide-based ammonium ylide-mediated epoxidations"
Johanna Novacek, Raphael Robiette, and Mario Waser*
Monatsh. Chem., 2017, 148, 77-81. [full text], opens an external URL in a new window
<2016>
43. "Bifunctional Ammonium Salt Catalyzed Asymmetric α-Hydroxylation of β-Ketoesters by Simultaneous Resolution of Oxaziridines"
Johanna Novacek, Joseph A. Izzo, Mathew J. Vetticatt, and Mario Waser*
Chem. Eur. J., 2016, 22, 17339-17344. [full text], opens an external URL in a new window
42. "New strategies and applications using electrophilic cyanide-transfer reagents under transition metal-free conditions"
Johannes Schörgenhumer and Mario Waser*
Org. Chem. Front., 2016, 3, 1535-1540. [full text], opens an external URL in a new window
41. "Phase-Transfer Catalysis with Ionene Polymers"
Maximilian Tiffner, Katharina Zielke, Judith Mayr, Marleen Häring, David Díaz Díaz,* and Mario Waser*
ChemistrySelect, 2016, 1, 4030–4033. [full text], opens an external URL in a new window
40. "Towards a General Understanding of Carbonyl-Stabilised Ammonium Ylide-Mediated Epoxidation Reactions"
Johanna Novacek, Lukas Roiser, Katharina Zielke, Raphaël Robiette,* and Mario Waser*
Chem. Eur. J., 2016, 22, 11422-11428. [full text], opens an external URL in a new window
39. "Benzylic Ammonium Ylide-Mediated Epoxidations"
Lukas Roiser, Raphaël Robiette,* and Mario Waser*
Synlett, 2016, 27, 1963-1968. [full text], opens an external URL in a new window
38. "Asymmetric tandem hemiaminal-heterocyclization-Aza-Mannich reaction of 2-formylbenzonitriles and amines using chiral phase transfer catalysis: an experimental and theoretical study"
Amedeo Capobianco,* Antonia Di Mola, Valentina Intintoli, Antonio Massa, Vito Capaccio, Lukas Roiser, Mario Waser, and Laura Palombi*
RSC Adv., 2016, 6, 31861-31870. [full text], opens an external URL in a new window
37. "Transition metal-free dimerization of alkynes using hypervalent iodine reagents"
Johannes Schörgenhumer and Mario Waser*
Tetrahedron Lett., 2016, 57, 1678-1680. [full text], opens an external URL in a new window
36. "Asymmetric α-Chlorination of β-Ketoesters Using Bifunctional Ammonium Salt Catalysis"
Johanna Novacek, Uwe Monkowius, Markus Himmelsbach, and Mario Waser*
Monatsh. Chem., 2016, 147, 533–538. [full text], opens an external URL in a new window
<2015>
35. "Bifunctional Phase Transfer Catalysis in the Asymmetric Synthesis of Biologically Active Isoindolinones"
Antonia Di Mola, Maximilian Tiffner, Francesco Scorzelli, Laura Palombi, Rosanna Filosa, Paolo De Caprariis, Mario Waser,* and Antonio Massa*
Beilstein J. Org. Chem., 2015, 11, 2591–2599. [full text], opens an external URL in a new window
34. "An organocatalytic biomimetic strategy paves the way for the asymmetric Umpolung of imines"
Mario Waser* and Johanna Novacek,
Angew. Chem., 2015, 127, 14434–14437, Angew. Chem. Int. Ed., 2015, 54, 14228–14231. [full text], opens an external URL in a new window
33. "Design of Chiral Urea-Quaternary Ammonium Salt Hybrid Catalysts for Asymmetric Reactions of Glycine Schiff Bases"
Maximilian Tiffner, Johanna Novacek, Alfonso Busillo, Katharina Gratzer, Antonio Massa,* and Mario Waser*
RSC Adv., 2015, 5, 78941-78949. [full text], opens an external URL in a new window
32. "Towards an asymmetric organocatalytic α-cyanation of β-ketoesters"
Raghunath Chowdhury, Johannes Schörgenhumer, Johanna Novacek, and Mario Waser*
Tetrahedron Lett., 2015, 56, 1911-1914. [full text], opens an external URL in a new window
31. "Asymmetric Syntheses of Three-Membered Heterocycles Using Chiral Amide-Based Ammonium Ylides"
Mathias Pichler, Johanna Novacek, Raphaël Robiette, Vanessa Poscher, Markus Himmelsbach, Uwe Monkowius, Norbert Müller and Mario Waser*
Org. Biomol. Chem., 2015, 13, 2092-2099. [full text], opens an external URL in a new window
<2014>
30."Stereoselective Cyclization Reactions under Phase-Transfer Catalysis"
Richard Herchl and Mario Waser*
Tetrahedron, 2014, 70, 1935-1960. [full text], opens an external URL in a new window
29. "Syntheses and Applications of (Thio)-Urea Containing Chiral Quaternary Ammonium Salt Catalysts"
Johanna Novacek and Mario Waser*
Eur. J. Org. Chem, 2014, 802-809. [full text], opens an external URL in a new window
28. "Thin-layer chromatography-spray mass spectrometry: A method for easy identification of synthesis products and UV filters from TLC aluminum foils"
Markus Himmelsbach*, Mario Waser and Christian Klampfl
Anal. Bioanal. Chem., 2014, 406, 3647-3656. [full text], opens an external URL in a new window
<2013>
27. "Towards Tartaric Acid-Derived Asymmetric Organocatalysts"
Katharina Gratzer, Guddeangadi N. Gururaja and Mario Waser*
Eur. J. Org. Chem, 2013, 4471-4482. [full text], opens an external URL in a new window
26. "Application Scope and Limitations of TADDOL-Derived Chiral Ammonium Salt Phase-Transfer Catalysts"
Guddeangadi N. Gururaja, Richard Herchl, Antonia Pichler, Katharina Gratzer, and Mario Waser*
Molecules, 2013, 18, 4357-4372. [full text], opens an external URL in a new window
25. "Asymmetric cyclopropanation of chalcones using chiral phase-transfer catalysts."
Richard Herchl and Mario Waser*
Tetrahedron Lett., 2013, 54, 2472-2475. [full text], opens an external URL in a new window
24. "Scope and limitations of diastereoselective aziridination reactions using stabilised ammonium ylides or α-bromo carbonyl nucleophiles."
Stefan Aichhorn, Guddeangadi N. Gururaja, Michael Reisinger and Mario Waser*
RSC Adv, 2013, 3, 4552-4557. [full text], opens an external URL in a new window
23. "Bifunctional Chiral Quaternary Ammonium Salt Catalysts – A Rapidly Emerging Class of Powerful Asymmetric Catalysts"
Johanna Novacek and Mario Waser*
Eur. J. Org. Chem, 2013, 637–648. [full text], opens an external URL in a new window
<2012>
22. "Investigations Concerning the Syntheses of TADDOL-Derived Secondary Amines and Their Use to Access Novel Chiral Organocatalysts"
Katharina Gratzer and Mario Waser*
Synthesis, 2012, 44, 3661-3670. [full text], opens an external URL in a new window
21. "Design, Synthesis, and Application of Tartaric Acid Derived N-Spiro Quaternary Ammonium Salts as Chiral Phase-Transfer Catalysts"
Mario Waser,* Katharina Gratzer, Richard Herchl, and Norbert Müller;
Org. Biomol. Chem., 2012, 10, 251-254. [full text], opens an external URL in a new window
20. "Photoreactive, water-soluble conjugates of hypericin with polyphosphazenes"
Ian Teasdale,* Mario Waser, Sandra Wilfert, Heinz Falk, Oliver Brüggemann;
Monatsh. Chem., 2012, 143, 355-360. [full text], opens an external URL in a new window
<2011>
19. "Process Development for a Key Synthetic Intermediate of LY2140023, a Clinical Candidate for the Treatment of Schizophrenia"
Mario Waser,* Eric D. Moher,* Sandy S. K. Borders, Marvin M. Hansen, David W. Hoard, Michael E. Laurila, Michael E. LeTourneau, Richard D. Miller, Michael L. Phillips, Kevin A. Sullivan, Jeffrey A. Ward, Chaoyu Xie, Cheryl A. Bye, Tanja Leitner, Brigitte Herzog-Krimbacher, Marcus Kordian, and Martin Müllner;
Org. Proc. Res. Dev., 2011, 15, 1266-1274. [full text], opens an external URL in a new window
18. "Identification of the Best-Suited Leaving Group for the Diastereoselective Synthesis of Glycidic Amides from Stabilised Ammonium Ylides and Aldehydes"
Richard Herchl, Martin Stiftinger, and Mario Waser;*
Org. Biomol. Chem., 2011, 9, 7023–7027. [full text], opens an external URL in a new window
17. "Progress in the Chemistry of Second Generation Hypericin Based Photosensitizers"
Mario Waser* and Heinz Falk;
Curr. Org. Chem., 2011, 15, 3894-3907.
16. "Molecular Editing and Assessment of the Cytotoxic Properties of Iejimalide and Progeny"
Julien Gagnepain, Emilie Moulin, Cristina Nevado, Mario Waser, Armin Maier, Gerhard Kelter, Heinz-Herbert Fiebig, and Alois Fürstner;*
Chem. Eur. J., 2011, 17, 6973–6984. [full text], opens an external URL in a new window
15. "Ammonium Ylides for the Diastereoselective Synthesis of Glycidic Amides"
Mario Waser,* Richard Herchl, and Norbert Müller;
Chem. Commun., 2011, 47, 2170-2172. [full text], opens an external URL in a new window
14. "Identification of Thymol Phase I Metabolites in Human Urine by Headspace Sorptive Extraction Combined with Thermal Desorption and Gas Chromatography Mass Spectrometry"
Bernhard Thalhamer,* Wolfgang Buchberger, and Mario Waser;
J. Pharm. Biomed. Anal., 2011, 56, 64–69.
<2005-2010>
13. "A Remarkable Cyclization of TADDOL-bisthioacetate under Oxidative Conditions"
Mario Waser,* Manuela Haunschmidt, and Markus Himmelsbach;
Monatsh. Chem., 2010, 141, 1347–1351.
12. "Development of a Scalable and Safe Process for the Production of 4-Chloro-2,3-Dimethylpyridine-N-oxide as a Key-Intermediate in the Syntheses of Proton Pump Inhibitors"
Mario Waser, Roland Obermüller, John Matthias Wiegand, Wolfgang Schiek, Hans Fierz, and Wolfgang Skranc;*
Org. Proc. Res. Dev., 2010, 14, 562-567.
11. "In Vitro Study of the Photocytotoxicity of Bathochromically Shifted Hypericin Derivatives"
Mieke Roelants, Bernd Lackner, Mario Waser, Heinz Falk, Patrizia Agostinis, Hendrik Van Poppel, and Peter A.M. de Witte;*
Photochem. Photobiol. Sci., 2009, 8, 822-829.
10. "A Versatile Protocol for Stille–Migita Cross Coupling Reactions"
Alois Fürstner,* Jacques-Alexis Funel, Martin Tremblay, Laure C. Bouchez, Cristina Nevado, Mario Waser, Jens Ackerstaff, and Christopher C. Stimson;
Chem. Commun., 2008, 2873-2875.
9. "Total Synthesis of Iejimalide A-D and Assessment of the Remarkable Actin-Depolymerizing Capacity of these Polyene Macrolides"
Alois Fürstner,* Cristina Nevado, Mario Waser, Martin Tremblay, Carine Chevrier, Filip Teplý, Christophe Aissa, Emilie Moulin, and Oliver Müller;
J. Am. Chem. Soc., 2007, 129, 9150-9161.
8. "Towards Second Generation Hypericin Based Photosensitizers for Photodynamic Therapy"
Mario Waser* and Heinz Falk;*
Curr. Org. Chem., 2007, 11, 547-558.
7. "Totalsynthesis of Iejimalide B"
Alois Fürstner,* Cristina Nevado, Martin Tremblay, Carine Chevrier, Filip Teplý, Christophe Aissa, and Mario Waser;
Angew. Chem. Int. Ed., 2006, 45, 5837-5842; Angew. Chem., 2006, 118, 5969-5974.
6. "Condensed Emodin Derivatives and Their Applicability for the Synthesis of a Fused Heterocyclic Hypericin Derivative"
Mario Waser* and Heinz Falk;*
Eur. J. Org. Chem., 2006, 1200-1206.
5. "9,12-Dibenzothiazolylhypericin and 10,11-Dibenzothiazolyl-10,11-didemethylhypericin: Photochemical Properties of Hypericin Derivatives Depending on the Substitution Site"
Mario Waser, Yulita Popova, Christian W. Klampfl, and Heinz Falk;*
Monatsh. Chem., 2005, 136, 1791–1797.
4. "Concerning Chemistry, Reactivity, and Mechanism of Transition Metal Catalysed Oxidation of Benzylic Compounds by Means of Ozone"
Mario Waser, Walther G. Jary, Peter Pöchlauer, and Heinz Falk;*
J. Mol. Catal. A-Chem., 2005, 236, 187-193.
3. "Syntheses, Photochemical Properties, and Tautomerism of Intramolecularly Friedel- Crafts Acylated Hypericin Derivatives"
Mario Waser, Yulita Popova, Christoph Etzlstorfer, Werner F. Huber, and Heinz Falk;*
Monatsh. Chem., 2005, 136, 1221–1231.
2. "An Efficient Regioselective Synthesis of Endocrocin and Structural Related Natural Anthraquinones Starting from Emodin"
Mario Waser, Bernd Lackner, Joachim Zuschrader, Norbert Müller, and Heinz Falk;*
Tetrahedron Lett., 2005, 46, 2377–2380.
1. "Intramolecularly Friedel-Crafts Acylated Emodin Derivatives. An Access to the Cores of Angucyclinones, Anthracyclinones, and to Hypericin Analogues"
Mario Waser and Heinz Falk;*
Monatsh. Chem., 2005, 136, 609–618.
6. "Asymmetric Phase‐Transfer Catalysis – From Classical Applications to New Concepts", opens an external URL in a new window
Jan Otevrel and Mario Waser
in Asymmetric Organocatalysis: New Strategies, Catalysts, and Opportunities. Łukasz Albrecht, Anna Albrecht, Luca Dell'Amico (Eds.) 2023, Chapter 3, 71-120, Wiley-VCH, ISBN 9783527832217.
5. "Tyrosine Alkaloids", opens an external URL in a new window
Uwe Rinner and Mario Waser
in From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products. A. Zografos (Ed.) 2016, Chapter 12, 431-472, Wiley, ISBN 978-1-118-75173-2.
4. "Monoterpenes and Iridoids", opens an external URL in a new window
Mario Waser and Uwe Rinner
in From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products. A. Zografos (Ed.) 2016, Chapter 6, 196-235, Wiley, ISBN 978-1-118-75173-2.
3. "Cooperative Catalysis Involving Chiral Ion Pair Catalysts", opens an external URL in a new window
Mario Waser, Johanna Novacek, and Katharina Gratzer
in Cooperative Catalysis. R. Peters (Ed.) 2015, Chapter 7, 197-226, Wiley-VCH Weinheim, ISBN 978-3-527-33689-0.
2. “Asymmetric Phase–Transfer Catalysis as a Powerful Tool in the Synthesis of Biologically Active Chiral Complex Natural Products“, opens an external URL in a new window
Guddeangadi N. Gururaja, Mario Waser
in Studies in Natural Product Chemistry. Atta-ur-Rahman (Ed.) 2014, 43, Chapter 14, 409-435, Elsevier.
1. "Asymmetric Organocatalysis in Natural Product Syntheses", opens an external URL in a new window
Mario Waser
Prog. Chem. Org. Nat. Prod., A. D. Kinghorn, H. Falk, J. Kobayashi (Eds), 2012, 96.
Springer, ISBN 978-3-7091-1162-8.
"Process for the manufacture of Isavuconazole or Ravuconazole"
Ruben van Summeren, Harrie Vaessen, Daniel Mink, and Mario Waser;
WO2014023623 A1.
"Iejimalid Analoga and Uses Thereof"
Alois Fürstner, Cristina Nevado-Blazquez, Mario Waser, Emilie Moulin, and Christophe Aissa;
WO2008/113320 A1.
"Isochalcogenourea-Catalyzed Activation of Allenoates"
4th Anatolian Conference on Organic Chemistry, Kemer, Turkey, March 6, 2024.
"Chiral Lewis Bases for Asymmetric Organocatalysis, opens an external URL in a new window"
Tetrahedron Seminar Series (online), Feb. 15, 2024.
"Chiral Isothioureas for Asymmetric Organocatalysis"
Keynote Lecture at the 27th International Electronic Conference on Synthetic Organic Chemistry, Nov. 2023.
"Chiral Lewis Bases for Asymmetric Organocatalysis"
Gakushuin University, Tokyo, Japan, Nov. 24, 2023.
"Chiral Isothio(seleno)urea-Catalyzed Activation of Allenoates"
IKCOC-15, Kyoto, Japan, Nov. 23, 2023.
"Chiral Lewis Bases for Asymmetric Organocatalysis"
Uni Göttingen, Göttingen, Germany, July 19, 2023.
"Chiral Onium Species for Asymmetric Organocatalysis"
Uni Leipzig, Leipzig, Germany, May 16, 2023.
"Chiral Onium Species for Asymmetric Organocatalysis"
TU Dresden, Dresden, Germany, May 15, 2023.
"Lewis base-catalyzed asymmetric synthesis of chiral oxygen-containing heterocycles using allenoates"
56th Bürgenstock Conference (Short Talk), Brunnen, Switzerland, May 10, 2023.
"Chiral Onium Species for Asymmetric Organocatalysis"
F. Schiller University, Jena, Germany, Jan. 18, 2023.
"(Thio)urea containing chiral ammonium salt catalysts"
Keynote Lecture at the 26th International Electronic Conference on Synthetic Organic Chemistry, Nov. 2022.
"Isoxazolidin-5-ones as a Platform for the Synthesis of b-Amino Acids"
EurJOC Lecture at the Blue Danube Symposium on Heterocylic Chemistry, Bratislava, Slovakia, Aug. 24, 2022.
"Chiral Onium Species for Asymmetric Organocatalysis"
University of Ljubljana, Slovenia, May 18, 2022.
"Chiral Onium Species for Asymmetric Organocatalysis"
University of Stockholm, Sweden, March 17, 2022.
"Chiral Ammonium Salt Ion Pairing Organocatalysis"
DosChem (Vienna Doctoral School in Chemistry) Retreat, Admont, Nov. 06, 2021.
"Design, Syntheses, and Applications of Chiral Bifunctional Ammonium Salt Catalysts"
21st Tetrahedron Symposium (online talk), June 23, 2021.
"From the Design of Chiral Quaternary Ammonium Salt Catalysts and Where it Lead us to"
Mulhouse (online talk), April 4, 2020.
"Asymmetric Syntheses Employing Chiral Quaternary Onium Salts"
FLOHET 2020, March 3, 2020, Gainesville, USA.
"Asymmetric Syntheses Using Chiral Quaternary Ammonium Salt Catalysts"
Binghamton University, Feb. 28, 2020, Binghamton, USA.
"Design, Synthesis, and Applications of Chiral Quaternary Ammonium Salts"
18th Austrian Chemistry Days, Sept. 24, 2019, Linz, A.
"Syntheses of Chiral Heterocycles Using Ammonium Ylides"
27th ISHC, Sept. 03, 2019, Kyoto, Japan.
"Asymmetric Syntheses and Catalysis Employing Chiral Quaternary (Amm)-Onium Salts"
University of Nagasaki, August 30, 2019, Nagasaki, Japan.
"Asymmetric Syntheses Using Chiral Quaternary Ammonium Salts"
Université de Rouen Normandie, April 26, 2019, Rouen, F.
"Asymmetric Syntheses Using Chiral Quaternary Ammonium Salts"
University of Caen, April 24, 2019, Caen, F.
"Synthesis and Catalysis Using (Chiral) Quaternary Ammonium Salts"
University Graz, January 17, 2019, Graz, A.
"Chiral Quaternary Ammonium Salts as Catalysts or Auxiliaries for Asymmetric Syntheses"
10th Singapore International Chemistry Conference (SICC-10), December 18, 2018, Singapore.
"Chiral Quaternary Ammonium Salts for Asymmetric Transformations"
TU Graz, October 10, 2018, Graz, A.
"Bifunctional Ammonium Salt-Catalysed Asymmetric a-Fluorination of b-Ketoesters"
22nd International Symposium on Fluorine Chemistry, July 23, 2018, Oxford, UK.
"Asymmetric Syntheses with Quaternary Ammonium Salts"
Comenius University, April 13, 2018, Bratislava, SK.
"Quaternary Ammonium Salts in Asymmetric Syntheses"
Palacky University, March 23, 2018, Olomouc, CZ.
"Quaternary Ammonium Salts in Asymmetric Syntheses"
Masaryk University, March 22, 2018, Brno, CZ.
"Quaternary Ammonium Salts for Asymmetric Syntheses"
LICAT-JKU Meeting, February 22, 2018, Linz, A.
“Chiral Bifunctional Phase-Transfer Catalysis – Catalyst Design and Applications”
2nd International Conference on Catalysis and Chemical Engineering, February 19, 2018, Paris, F.
“Quaternary Ammonium Salts for Asymmetric Syntheses”
Servier, February 13, 2018, Budapest, H.
“Chiral Quaternary Ammonium Salts for Asymmetric Syntheses”
University of Ghent, October 18, 2017, Ghent, BE.
“Design, Synthesis and Applications of Bifunctional Chiral Urea-Quaternary Ammonium Salt Catalysts”
20th European Symposium on Organic Chemistry (ESOC 2017), July 4, 2017, Cologne, DE.
“Chiral Quaternary Ammonium Salts as Catalysts and Auxiliaries for Asymmetric Transformations"
University of Strasbourg, February 9, 2017, Strasbourg, F.
“Chiral Quaternary Ammonium Salts as Catalysts and Auxiliaries for Asymmetric Transformations"
University of Basel, February 8, 2017, Basel, CH.
“Chiral Quaternary Ammonium Salts as Catalysts and Auxiliaries for Asymmetric Transformations"
University of Mulhouse, February 8, 2017, Mulhouse, F.
“Chiral Quaternary Ammonium Salts as Catalysts and Auxiliaries for Asymmetric Transformations"
Organic Syntheses Symposium @ University of Zürich, February 7, 2017, Zürich, CH.
“Design, Syntheses, and Applications of (Thio)-Urea/Quaternary Ammonium Salt Hybrid Catalysts"
21st International Conferenc on Organic Synthesis, December 12, 2016, Mumbai, India.
“Chiral Quaternary Ammonium Salts as Catalysts and Auxiliaries in Asymmetric Reactions"
BHABHA Atomic Research Centre, December 9, 2016, Mumbai, India.
“Quaternary Ammonium Salts as Powerful Catalysts and Auxiliaries for Asymmetric Reactions”
University Innsbruck, November 17, 2016, Innsbruck, A.
“Chiral Quaternary Ammonium Salts as Catalysts and Auxiliaries in Asymmetric Transformations”
TU Vienna, April 28, 2016, Vienna, A.
“Quaternary Ammonium Salts for Asymmetric Synthesis"
Anatolian Conference on Synthetic Organic Chemistry, March 23, 2016, Kusadasi, TR.
“Chiral Quaternary Ammonium Salts in Asymmetric Synthesis”
KickOff Meeting - Workgroup Organic Chemistry of the Austrian Chemical Society (GÖCH), March 1, 2016, Linz, A.
“Quaternary Ammonium Salts: Powerful Catalysts and Auxiliaries in Asymmetric Reactions”
LMU Munich, February 1, 2016, Munich, DE.
“Design, Syntheses, and Applications of (Thio)-Urea/Quaternary Ammonium Salt Hybrid Catalysts”
University of Oxford, November 26, 2015, Oxford, UK.
“Quaternary Ammonium Salts: Powerful Catalysts and Auxiliaries in Asymmetric Reactions”
University of Liverpool, November 25, 2015, Liverpool, UK.
“Quaternary Ammonium Salts: Powerful Catalysts and Auxiliaries in Asymmetric Reactions”
University of Bristol, November 23, 2015, Bristol, UK.
“Quaternary Ammonium Salts: Powerful Catalysts and Auxiliaries in Asymmetric Reactions”
University of Regensburg, November 18, 2015, Regensburg, DE.
“Design and Application of New Tools for (Asymmetric) Catalysis”
JKU Linz, June 29, 2015, Linz, A.
“Asymmetric Syntheses of Epoxides and Aziridines using Ammonium Ylides”
16th Blue Danube Symposium on Heterocyclic Chemistry, June 17, 2015, Balatonalmadi, H.
“Quaternary Ammonium Salts: Powerful Catalysts and Auxiliaries in Asymmetric Reactions”
University of Salerno, May 28, 2015, Salerno, I.
“Design, Syntheses, and Applications of Chiral Quaternary Ammonium Salt Catalysts”
University of Vienna, May 4, 2015, Vienna, A.
“Quaternary Ammonium Salts: Powerful Asymmetric Catalysts and Auxiliaries for Stereoselective Reactions”
DPx Fine Chemicals, Oct. 10, 2014, Linz, A.
“Design, Synthesis and Applications of Bifunctional (Thio)-Urea Containing Chiral Ammonium Salt Catalysts”
GDCH Chemiedozententagung, March 10-11, 2014, Paderborn, DE.
“From the Development of Tartaric Acid Derived Organocatalysts Towards New Methods for Asymmetric Synthesis"
Université catholique de Louvain, Jan. 27, 2014, Louvain-la-Neuve, BE.
“Bifunctional (Thio)-Urea Containing Chiral Ammonium Salt Catalysts”
Syngenta Crop Protection, Jan. 13, 2014, Stein, CH.
“From the Development of Tartaric Acid-Derived Organocatalysts Towards New Stereoselective Methods”
Austrian Chemistry Days, Sept. 23-25, 2013, Graz, AT.
"Syntheses of Epoxides and Aziridines Using Ammonium Ylides”
The 24th International Society of Heterocyclic Chemistry Congress, September 8-13, 2013, Shanghai, China.
“From the Development of Tartaric Acid-Derived Organocatalysts Towards New Synthetic Methodologies”
GDCH Chemiedozententagung, March 11-13, 2013, Berlin, DE.
"Design, Syntheses and Applications of TADDOL-Derived Asymmetric Phase-Transfer Catalysts”
4th EuCheMS Chemistry Congress, August 26-30, 2012, Prague, CZ.
“Design, Syntheses, and Applications of TADDOL-Derived Asymmetric Phase-Transfer Catalysts”
Poster Chalk-Talk - Gordon Research Conference on Stereochemistry, July 29 - Aug 3, 2012, Newport, USA.
“Tartaric Acid Derived Organocatalysts”
14th Austrian Chemistry Days, Sept. 26-29, 2011, Linz, Austria; OP-11.
“Synthesis of TADDOL-derived N-spiro Quaternary Ammonium Salts and Applications in Asymmetric Phase-Transfer Catalysis”
Gregynog Synthesis Workshop, Sept. 23-25, 2011, Newtown, UK.
“Towards Tartaric Acid Derived Quaternary Ammonium Salts for Asymmetric Phase Transfer Catalysis”
17th European Symposium on Organic Chemistry (ESOC 2011), July 10-15, 2011, Crete, Greece; OC-25.
“Towards Tartaric Acid Derived Organocatalysts”
3rd Young Investigator's Workshop of the EuCheMS Organic Division, July 8-9, 2011, Heraklion, Greece.
“TADDOL Derived Quaternary Ammonium Salts: Syntheses and Applications in Asymmetric Catalysis”
XXIII Conference on Advances in Organic Synthesis (CAOS 2011), June 26-30, 2011, Hradec Kralove, Czech Republic.
“Totalsyntheses of the Iejimalides”
IKCOC-10, Nov. 13-17, 2006, Kyoto, Japan.
“Towards Second Generation Hypericin Based Photosensitizers”
10th World Congress of the International Photodynamic Association, June 22-25, 2005, Munich, Germany.