Research emphasis of the research at the Institute for Physiology and Pathophysiology are the mechanisms of cardiovascular calcifications, especially medial vascular calcification (Monckeberg's arteriosclerosis).
This calcification is defined as deposition of calcium-phosphate in the medial layer of the arteries. This type of calcification differs from atherosclerosis, which is characterized by intimal atherosclerotic plaque formation. While atherosclerosis is related to “traditional” risk factors, medial vascular calcification is also fostered by “non-traditional” risk factors, such as phosphate.
Medial vascular calcification occurs during the aging process, but is strongly accelerated in diabetes mellitus and chronic kidney disease. Furthermore, some genetic conditions can cause vascular calcification. While this calcification was considered for long time as a degenerative process without medical relevance, the current research indicates that vascular calcification is associated with an increased risk for cardiovascular events, such as stroke and myocardial infarction. The underlying mechanisms for this association and a causal relationship is yet not clearly established. Vascular calcification may cause stiffening of the arteries, which may lead to cardiac hypertrophy and diastolic dysfunction. Furthermore, vascular calcification may impair autoregulation of organ perfusion and modify atherosclerotic plaque stability.
Ectopic cardiovascular calcifications were recognized as an active and regulated process, In medial vascular calcification, vascular smooth muscle cells may play a decisive role. However, the underlying mechanisms are not clearly defined, and no broadly applicable therapeutic treatment is available. Further research is required, to understand the mechanisms and relevance of vascular calcifications, in order to translate current concepts into new therapeutic concepts, that may benefit cardiovascular survival.
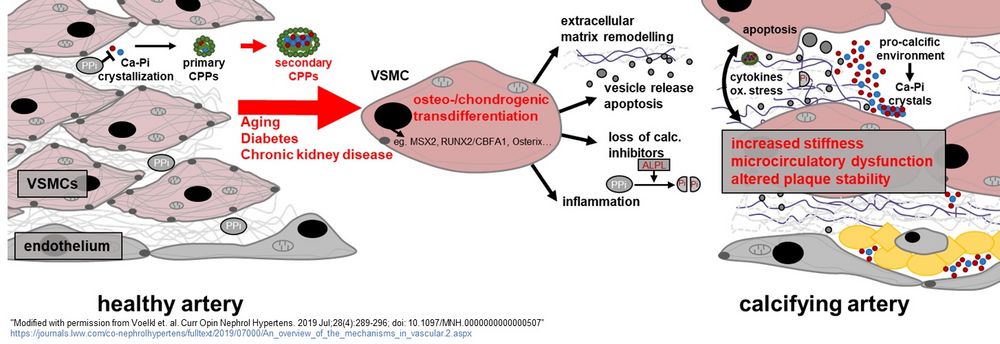 Figure 1: Putative mechanisms and sequaelae of vascular calcification
("Modified with permission from Voelkl et. al. Curr Opin Nephrol Hypertens. 2019 Jul;28(4):289-296; doi: 10.1097/MNH.0000000000000507); https://journals.lww.com/co-nephrolhypertens/fulltext/2019/07000/An_overview_of_the_mechanisms_in_vascular.2.aspx
Figure 1: Putative mechanisms and sequaelae of vascular calcification
("Modified with permission from Voelkl et. al. Curr Opin Nephrol Hypertens. 2019 Jul;28(4):289-296; doi: 10.1097/MNH.0000000000000507); https://journals.lww.com/co-nephrolhypertens/fulltext/2019/07000/An_overview_of_the_mechanisms_in_vascular.2.aspx
Phosphate is added in increasing amounts in the course of industrial food processing (eg E-339) nowadays. A healthy human can most likely compensate this phosphate load by renal excretion of phosphate. In addition, our body produces mineralization inhibitors, that are able to prevent ectopic crystallization of calcium and phosphate. This includes eg. the formation of pyrophosphate and endogenous proteins such as fetuin-A and matrix GLA protein. Growth of calcium-phosphate crystals may be prevented by formation of calcium-phosphate-protein particles (CPPs). If this “anti-calcification defence” is weakened or overloaded, ectopic mineralization and calcifications may occur.
Especially in chronic kidney disease, hyperphosphatemia may occur, which has been recognized as a very potent stimulator of vascular calcification. Paradoxically, chronic kidney disease causes demineralization of the bone and a mineralization of the vasculature. Some indications exist, that phosphate load may also be disadvantageous in humans without chronic kidney disease. A focus of our research is to understand regulations and dysregulations of the phosphate homeostasis, as well as putative detrimental sequalae of phosphate load such as cardiac hypertrophy and vascular stiffness.
Phosphate, but also hyperglycaemia and inflammatory mediators, may be sensed by vascular smooth muscle cells. These cells show the capability to transform their phenotype, and can take up characteristics of osteoblasts and chrondroblasts. These transdifferentiated smooth muscle cells are able to impair endogenous mineralization inhibitors, degrade and remodel the extracellular matrix, promote inflammatory processes, and increasingly tend to undergo apoptosis, as well as cellular vesicle release.
These processes may promote an active calcification of the vascular wall. A complex network of cellular signalling cascade orchestrates the osteo-/chondrogenic transdifferentiation. A therapeutic approach modifying key signalling nodes of these signalling pathways could provide a feasible treatment to prevent vascular calcification, and lower cardiovascular mortality of the patients at risk. A research focus of our department is to investigate inflammatory signalling pathways that promote vascular calcification and to translate these insights into new therapeutic concepts.
We are very grateful to the funding agencies, that allowed us to conduct our research: